News
BeyondDx: “Blood-Based Early Detection of Cancer & Mental Disorders — Year One of Commercialization & Global Expansion”
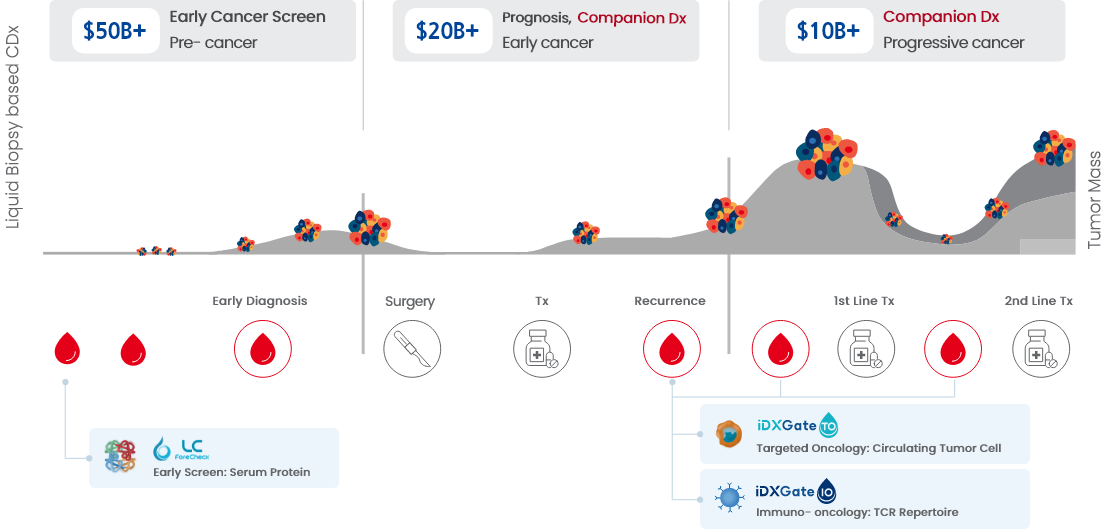
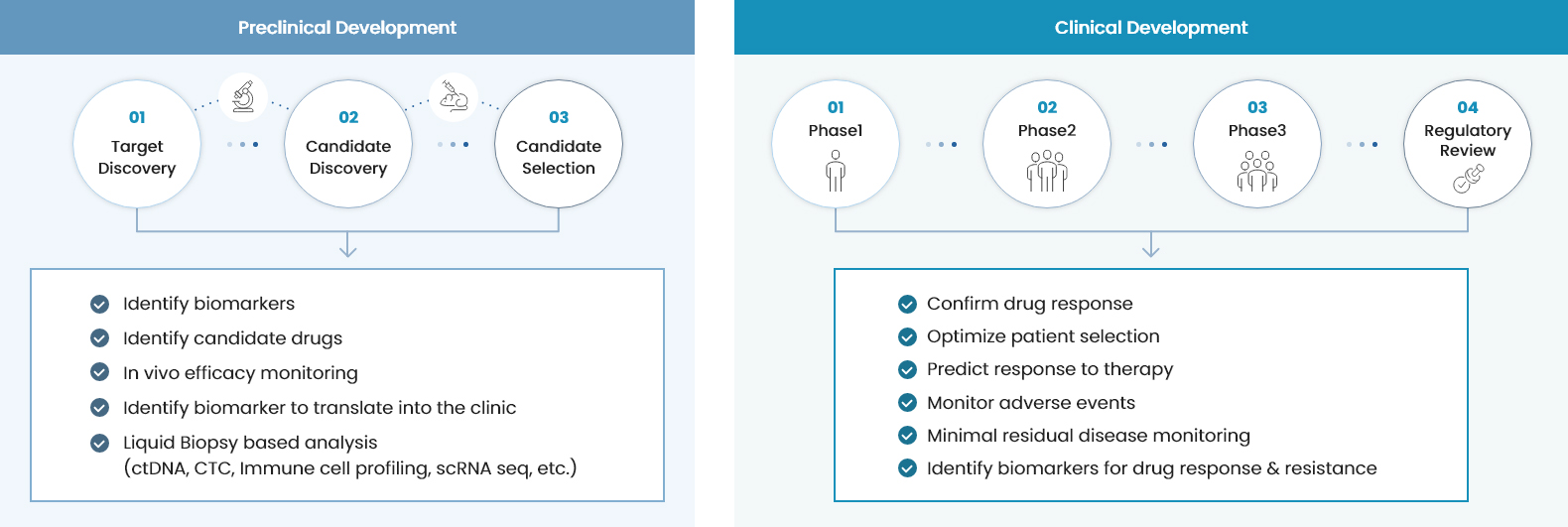
https://www.thebionews.net/news/articleView.html?idxno=21436BeyondDx: “Early Detection of Cancer and Mental Disorders Through Blood Tests…2026 to Be a Year of Commercialization and Global Expansion”기자명 Kang, In Hyo 입력 2026.01.12 16:20 댓글 0페이스북(으)로 기사보내기 트위터(으)로 기사보내기 URL복사(으)로 기사보내기 기사저장 다른 공유 찾기 - [Interview] BeyondDx Founder and CEO Jung So-jin- BeyondDx\'s ‘lifecycle blood diagnostics’… Spanning early detection through MRD monitoring- Aiming for domestic approval of ‘ForeCheck LC’ this year following a pivotal clinical trial in Korea, while advancing exploratory clinical trials of ‘ForeCheck MDD’ in ChinaJung So-jin, founder and CEO of BeyondDx, poses during an interview with THE BIO. (Source: THE BIO DB)[by Kang, In Hyo] \"This year, we aim to secure domestic approval for \'ForeCheck LC,\' a liquid biopsy-based assay for early lung cancer screening, one of our core pipeline assets. We are also preparing to initiate an \'exploratory clinical trial\' in China within the year for \'ForeCheck MDD,\' an early diagnostic test for major depressive disorder. Accordingly, we expect to achieve tangible progress in our global expansion efforts.\"Jung So-jin, founder and CEO of BeyondDx, made these remarks in a recent interview with <THE BIO>. She drew notable attention last year after receiving the grand prize at the Women\'s Startup Competition hosted by the Ministry of SMEs and Startups, in recognition of her development of a lung cancer diagnostic technology that applies a combinatorial algorithm integrating multiple blood-based biomarkers.BeyondDx, a multi-integrated analytical solution developer marking its third anniversary this year, has designated 2026 as a pivotal year for commercialization and global expansion. On January 6, THE BIO met with Jung at the company’s headquarters in Gwangmyeong, Gyeonggi Province, to discuss BeyondDx’s key accomplishments over the past year and to examine its business goals for the year ahead.Founded in August 2023, BeyondDx is a diagnostic technology company focused on the development of customized, multi-integrated blood-based analytical solutions. Leveraging proprietary technology that precisely identifies disease characteristics through the integrated analysis of trace-level blood ‘biomarkers,’ the company is building a specialized pipeline targeting oncology and mental disorders. Its flagship programs include ‘ForeCheck LC (early diagnosis of lung cancer)’ and ‘ForeCheck MDD (diagnosis of major depressive disorder). Both programs seek to enable early disease screening through the analysis of minimal blood samples.\"BeyondDx\'s vision is to \'save lives through in vitro diagnostics using a single drop of blood,\'\"Jung stated. \"Our mission, reflected in the name BeyondDx, signifies our aspiration to become \'a next-generation diagnostics company that goes beyond conventional diagnosis.\'\"BeyondDx is developing a pipeline portfolio designed to address the entire disease lifecycle. Broadly, this portfolio is structured into three categories: ‘early diagnosis’ assays intended to identify diseases prior to the onset of clinical symptoms, ‘prognosis prediction’ tests that, following diagnosis at the symptomatic stage, assess treatment response and prognosis, and ‘monitoring’ assays aimed at surveillance to detect disease recurrence or confirm sustained remission after treatment.Source: BeyondDxAt present, the pipeline in the most advanced stages of development is its early diagnostic testing. Within the segment, ForeCheck LC is targeting commercialization in Korea within the year, while ForeCheck MDD is pursuing entry into the global market, supported by the exploratory clinical trial in China.\"In the field of early disease detection, both oncology and psychiatric indications rely on the identification of specific protein biomarkers present in blood. Our focus on protein biomarkers stems from their high biological relevance, as such markers are more likely to reflect underlying pathogenic processes and generate detectable signals even at very early disease stages, prior to the onset of symptoms,\" Jung explained.Accordingly, BeyondDx has established its early diagnostic pipeline around protein-based biomarkers. \"At the early stage of diagnosis, clinical symptoms are generally absent, which makes it difficult to identify disease presence using a single indicator and inevitably constrains diagnostic accuracy. To overcome these limitations, we are moving beyond reliance on a single biomarker and instead selecting and applying multiple highly specific biomarkers that more comprehensively represent the disease,\" Jung emphasized.\"We are improving diagnostic accuracy by analyzing the expression patterns of multiple biomarkers through machine learning (ML) and artificial intelligence (AI)-based algorithms. We believe that this integrated approach, combining multi-biomarker profiling with advanced algorithms, will constitute a core source of competitive advantage in the field of early disease detection,\" she further commented.In the field of blood-based companion diagnostics, conventional approaches employed by existing companies predominantly involve the isolation of disease-associated circulating tumor DNA (ctDNA) from blood samples, followed by high-depth sequencing to profile underlying genetic mutations. Although this strategy enables indirect assessment of molecular features of cancer, it has been criticized for inherent limitations, particularly its reduced capacity to accurately capture tumor heterogeneity and to reflect treatment responses in real time.In contrast, BeyondDx\'s early diagnosis mechanism emphasizes the direct capture and analysis of circulating tumor cells (CTCs) from blood, alongside ctDNA assessment. Jung explained that profiling based on intact cancer cells enables a more accurate identification of cancer-specific biological associations and provides a direct reflection of tumor characteristics. \"This approach establishes a robust foundation for more precise evaluation of cancer treatment potential and real-time monitoring of treatment response,\" she said.BeyondDx plans to expand this methodological approach to prognostic testing. \"Cancer undergoes continuous genetic mutations and evolves during tumor progression and metastasis, giving rise to \'neoantigens\' that are increasingly recognized as key indicators of treatment response and prognosis. Our company intends to develop an analytical platform capable of sensitively detecting and tracking these neoantigen dynamics and to apply it to our prognostic testing pipeline,\" Jung further explained.BeyondDx is also advancing pipeline expansion in the area of post-treatment monitoring diagnostics. Following treatment intervention, tumors are frequently eliminated or substantially reduced, requiring highly sensitive analytical methods capable of accurately detecting residual cancer-related signals that may remain at extremely low levels.\"In this context, our company is adopting an analytical approach centered on the T-cell receptor (TCR). Immune cells function as the body\'s surveillance system and are highly sensitive to external antigens and tumor-associated mutations. Among these, T cells play a central role in the direct recognition of and response to antigens, and the composition and expression patterns of T-cell receptors dynamically change, either increasing or decreasing, in response to changes in antigens or mutations,\" Jung remarked.\"By harnessing these immunological features, BeyondDx is expanding its TCR profiling technology into post-treatment monitoring assays that evaluate residual disease after treatment. This approach will be applied to high-precision monitoring solutions, including minimal residual disease (MRD) testing,\" she added.“Our company is developing a customized diagnostic and monitoring testing system that reflects the distinct characteristics of each disease stage, from the pre-onset phase through post-treatment management,” Jung emphasized. “By tailoring the selection of biomarkers according to disease type and clinical purpose, we aim to achieve optimized improvements in diagnostic accuracy on a case-by-case basis.”Source: BeyondDxExpectations are particularly high for the company’s most advanced pipeline asset, the early lung cancer screening test ‘ForeCheck LC.’ \"ForeCheck LC has identified three highly specific biomarkers for lung cancer and has improved diagnostic accuracy through the application of machine learning. We are currently preparing to initiate full-scale clinical trials within this year,\" Jung commented.Conventionally, the performance of lung cancer diagnostic technologies is evaluated by comparing a ‘healthy control cohort’ with a ‘lung cancer patient cohort,’ based on metrics like sensitivity, specificity, and overall predictive accuracy. These evaluation standards have served as the primary criteria for regulatory approval by the Ministry of Food and Drug Safety (MFDS) and the U.S. Food and Drug Administration (FDA).However, both regulatory authorities and real-world clinical practice increasingly emphasize the need to define a more ‘clinically relevant high-risk population.’ In this context, the MFDS and the U.S. FDA jointly recommend assessing the discriminatory performance of low-dose chest computed tomography (LDCT), the standard protocol for lung cancer screening. This evaluation aims to determine how effectively LDCT differentiates lung cancer from non-lung cancer in high-risk populations presenting with pulmonary nodules.For the interpretation of LDCT scans, the Lung Imaging Reporting and Data System (Lung-RADS), established by the American College of Radiology (ACR), is widely applied. Although a Lung-RADS score of 2 or higher indicates the presence of a pulmonary nodule, the majority of such findings are ultimately determined to be benign. On this basis, BeyondDx conducted an analysis using the ‘high-risk group with pulmonary nodules’ classified as 2 or higher as the control group. The results demonstrated that, even among patients with Stage 1 lung cancer, both sensitivity and specificity exceeded 80%. According to Jung, these findings were generated from exploratory clinical studies.Ahead of its planned clinical trial scheduled for this year, BeyondDx intends to gradually broaden its patient cohort. The initial phase will prioritize the enrollment of patients at high risk for lung cancer, followed by an expansion of its ability to differentiate between lung cancer and other non-malignant pulmonary diseases, including tuberculosis, asthma, and pneumonia.In addition, BeyondDx plans to extend its analyses to other malignancies, including stomach, prostate, colorectal, pancreatic, and liver cancers, which represent the five most common cancers in Korea, to further validate its cancer differentiation capability. Through this expanded evaluation, the company aims to more rigorously demonstrate diagnostic performance that is directly applicable to real-world clinical practice.\"The company\'s target diagnostic performance is to achieve a sensitivity of at least 85% and a specificity of 90%. Based on data obtained from more than 2,000 \'exploratory clinical trials\' in Korea and the application of machine learning algorithms, we anticipate meeting these performance targets without difficulty,\" Jung remarked. \"We plan to initiate discussions regarding regulatory approval in the first half of this year, and, if all goes well, we expect to obtain approval by the end of the year.\"\"ForeCheck MDD has completed biomarker validation and analytical performance assessments through domestic clinical studies. Based on these results, we are currently preparing to initiate small-scale exploratory clinical trials in Korea,\" she mentioned.\"In China, driven by increasing interest in depression diagnostic technologies, we are conducting joint research and clinical studies in collaboration with two institutions (Luye Diagnostics and Shanghai Kehua Bio-Engineering). Clinical trials in China are currently at the research stage, and we are preparing for an exploratory trial involving approximately 250 participants this year in collaboration with a hospital in Hangzhou,\" Jung further emphasized.(From the left) Kang Hyuk-shin, Vice President of Shanghai Kehua Bio-Engineering, and Lee Gwang-hyouk, Vice President of BeyondDx, pose for a commemorative photograph following the signing of an MOU. (Source: BeyondDx)BeyondDx plans to commercialize ForeCheck LC domestically within this year, with its primary deployment focused on health screening centers as well as primary and secondary medical institutions. To this end, the company is currently in discussions with five major screening centers in the Seoul metropolitan area and is concurrently positioning large-scale primary and secondary healthcare institutions as key partners for market entry.\"Many primary and secondary medical institutions do not operate in-house CT scanners, but we can establish a system that identifies high-risk individuals, performs blood tests, and collaborates with screening centers under a contractual framework,\" Jung explained. \"ForeCheck LC is positioned not as a diagnostic model directly linked to a specific treatment or pharmaceutical product, but as a screening test applicable to all individuals aged 50 and older who are eligible for cancer screening.\" In other words, health screening centers and primary and secondary medical institutions, where asymptomatic members of the general population predominantly undergo routine health screenings, are the company’s core target user base.By contrast, LDCT is employed as the standard modality for lung cancer screening in tertiary medical institutions. However, it is associated with a relatively high false-positive rate. “In actual clinical practice, although a large number of subjects may be flagged as suspected lung cancer cases, only a limited proportion are ultimately confirmed to have cancer. Therefore, BeyondDx anticipates that the use of blood-based assays as an adjunct to LDCT could contribute to reducing unnecessary follow-up examinations and mitigating overdiagnosis,” Jung said.Source: BeyondDxBeyondDx is developing two diagnostic pipelines in parallel: a ‘prognostic test’ designed to predict treatment response and curative potential in patients following diagnosis, and a ‘monitoring (MRD) test’ intended to assess residual disease and detect potential recurrence after treatment. Both programs are currently in the clinical research phase. \"These initiatives are being developed under the framework of the \'DeepTech TIPS\' project, which has been underway since July 2024, and are focused on colorectal, liver, and kidney cancers,\" Jung explained.BeyondDx is pursuing Series A financing this year, targeting a total raise of KRW 5 billion (approximately USD 3.4 million). Shortly after its founding, the company secured seed funding in December 2023 from Bluepoint Partners, a leading accelerator (AC) in Korea. BeyondDx is aiming for an initial public offering (IPO) for 2028-2029 and anticipates a transition to a full-scale revenue growth phase following the commercialization of its flagship pipeline, ForeCheck LC, in 2027 (estimated sales of KRW 10.1 billion).\"In the in vitro diagnostic field, it is uncommon to secure data from more than 2,000 cases solely during the exploratory clinical trial phase, as achieved with ForeCheck LC. We have already accumulated clinical data from over 2,000 cases through exploratory clinical studies, and based on this foundation, we are preparing to conduct a large-scale clinical trial involving approximately 2,000 participants to support regulatory approval,\" Jung stated.Finally, Jung remarked, \"Most diseases cannot be explained by a single cause or mechanism but rather arise from a complex interplay of multiple factors. In particular, as disease severity and the intractable nature of diseases increase, substantial unmet needs inevitably persist both within individual patients and across patient populations.\" She added, \"To maximize therapeutic effectiveness, a more comprehensive approach is necessary. All of the company\'s R&D strategies are aligned to address these unmet medical needs.\"Jung projected that these changes will increasingly expose the inherent limitations of conventional single-marker diagnostic approaches and drive growing demand for next-generation diagnostic solutions. \"Looking ahead, blood-based testing will become the standard for assessing the risk of developing depression and for the continuous monitoring of disease progression. BeyondDx is committed to developing a diagnostic platform that is easily and quickly accessible to patients, while enabling healthcare professionals to make more timely and informed clinical decisions,\" she added.\"Our company is developing a diagnostic service that allows anyone to undergo testing at a time of their choosing without undue burden. Successfully implemented in real-world clinical and market settings, this approach is expected to have a significant impact,\" Jung further stated. Kang, In Hyo (zenith@thebionews.net)
2026-01-12