
(From left in the photo) Guo Sheng Min, CEO of Senlang Bio, and Jung Sojin, CEO of BeyondDx, taking a commemorative photo after signing the partnership agreement. / Photo by BeyondDx.
BeyondDx Inc., led by CEO Jung Sojin, has announced that on the 3rd, it has signed a partnership agreement with Senlang Bio, a cell therapy specialist in China's Sichuan Science Special Zone, for the development of patient-specific cell therapy.
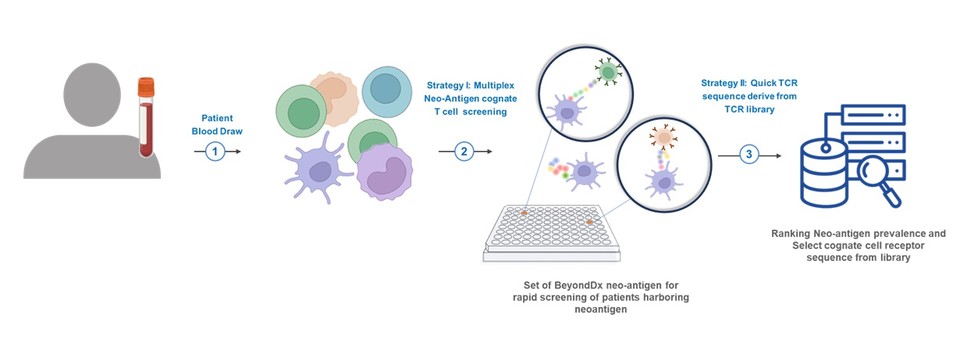
This partnership is based on the establishment of a strategic cooperation system for the development, production, and application of personalized treatment solutions tailored to various cancer environments for each patient. By utilizing Senlang Bio's technology for developing personalized cell therapy and its clinical cooperation system with more than 20 large hospitals in the Sichuan Science Special Zone, BeyondDx aims to quickly build and apply a treatment system that matches the characteristics of individual patients by applying the 'iDxGate IO technology' that captures and profiles T cells that respond to cancer immune environments. Additionally, this collaboration will include the discovery of neoantigens in resistant cancer types, construction of TCR libraries for them, development, production, and commercialization of therapies, and will progress in sharing mid-to-long-term milestones.
BeyondDx is a diagnostic company based on liquid biopsy, which was established in August with a focus on unmet needs in disease and the market. The most significant point of differentiation from existing liquid biopsy companies is that, although they use blood as a specimen, they combine all forms of biomarkers present in the blood (such as protein biomarkers, circulating tumor cells, circulating tumor DNA, T cells, etc.) according to the disease and application purpose to build the optimal diagnostic system.
This partnership with Senlang Bio involves the iDXGate IO (TCR profiling) solution, a diagnostic platform it possesses. This solution allows for the rapid profiling of the characteristics of T cells (neo-antigen response TCR combination) that respond to cancer environments, making it applicable to T cell-based personalized treatments.
Senlang Bio, a cell therapy specialist, was established in 2016 and operates a clinical pipeline with more than 20 different forms. In August of last year, its CD7-CAR-T cell therapy 'SENL101 (development code)' was designated as an orphan drug by the U.S. Food and Drug Administration (FDA), proving its technological capabilities.
Jung Sojin, CEO of BeyondDx, stated, "While cancer is being controlled according to systematic treatment guidelines, personalized treatment tailored to the individual patient's condition is necessary for cases that have progressed to resistant advanced-stage cancer." She added, "iDXGate IO, which effectively interprets profiling of neoantigens representing an individual patient's cancer environment and immune response, is expected to be a meaningful alternative for a new immunotherapy approach." Jeong also explained that, through this partnership with Senlang Bio, they plan to expand the platform to target various types of cancer, beyond clinical utility verification.
Guo Sheng Min, CEO of Senlang Bio, mentioned, "We are currently conducting clinical research on CAR-T pipelines with more than 20 major hospitals and patient-specific IIT clinical trials." He added, "In cell therapy approaches for cancer, there have been limitations in the interpretation of neoantigens for individual patients. Through this partnership, we expect that personalized approaches will rapidly advance, along with securing customized neoantigens and the application of the high-efficiency CAR screening technology 'CDDP™'."